Implement a Compliant, Scalable Quality Management System
ISO 13485 | ISO 9001 | ICMED | IMDR


Why a Robust QMS is Essential?


Mandatory for Regulatory Compliance
Prepares You for Audits & Inspections
Customer Confidence & Quality Culture
Reduces Risk & Variability
Key Benefits


A QMS is not limited to a specific device or product class — it is a company-wide system. It governs how your organization plans, controls, documents, and improves quality across design, production, procurement, servicing, and post-market activities.
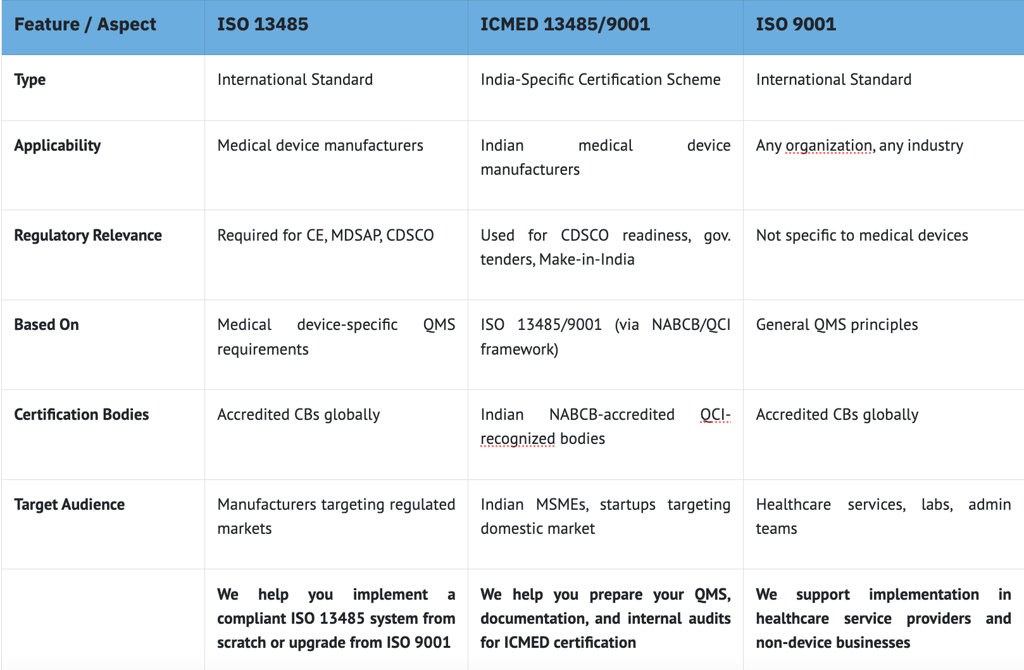
Which QMS Standard is Right for You?
Whether you’re targeting exports, government tenders, or internal process improvement — the right QMS standard makes all the difference. Here's a quick side-by-side breakdown to help you decide based on your product, market, and certification goals.
How We Can Help?
End-to-end support for QMS design, implementation, compliance, and continual improvement.
QMS Implementation & Documentation
Help you build your QMS from scratch
Gap analysis and reviewing your existing QMS
Quality Manual, SOPs, and Work Instructions
Process mapping and risk-based controls
Document control, record formats, templates
Certification Planning & Audit Preparation
Selection of appropriate QMS standard and certification body
Creation of certification roadmap and timeline
Alignment with ISO 13485 / ICMED 13485 / ISO 9001 requirements
Review of readiness documents (MRs, audit plans, checklists, mandatory procedures)
Internal audit planning and execution
Corrective & preventive action (CAPA) tracking
Closure of non-conformities and documentation updates
Mock audits for ISO/ICMED readiness
Internal Audit & Non-Conformity Closure
QMS Enhancement & Continual Improvement Support
Periodic document review and update
Support for management review preparation
Improving CAPA closure effectiveness
Risk-based revision of processes as needed
Key Deliverables
Quality Manual, Quality Policy & Quality Objectives
Standard Operating Procedures (SOPs) & Work Instructions
Document Control Formats & Record Templates
Process Maps & Risk-Based Controls
Risk Management File (if applicable)
QMS Documentation Support (as per IMDR 2017 – Fifth Schedule)
Master List of Documents and Records
Design and Development Documentation (if applicable)
Internal Audit & Management Review Records
End-to-end Implementation Support
Audit-Ready QMS Documentation
Certification Body Audit Support
Post-market Surveillance setup
Ongoing Regulatory Support
Who We Help?
First-Time Applicants - building QMS from scratch
Manufacturers Without In-House Regulatory Teams
Whether you're a Startup, SME, or an established manufacturer, we tailor our support to meet your specific product, process, and team needs.
Companies Transitioning from ISO 9001 to ISO 13485
OEMs & Contract Manufacturers
Companies applying for government tenders or GEM portal listings
Startups & Innovation-Driven Enterprises
Established Manufacturers Seeking Market Expansion
Exporters & Importers Handling CDSCO or Foreign Market Approvals
Ready to Implement a strong Quality Management System?
Let’s build a system that works for your team — not just your certificate.
Why Choose VIDHA?
Expertise You Can Trust
Hands-on experience, backed by a strong understanding of global and Indian regulatory frameworks.
Personalized Solutions
We tailor every document, process, and support plan to your specific device class, regulatory pathway, and business goals.
Reliable & Confidential Support
We prioritize confidentiality, integrity, and compliance — ensuring your data and submissions are handled with care and professionalism.
Our services are focused, efficient, and aligned with your timelines and budgets.
Timely & Cost-Effective Delivery