
MedQMS Pro –
ERP + QMS for Medical Device Manufacturers
ISO 13485–Aligned ERP + QMS
A purpose-built digital platform for medical device and healthcare equipment manufacturers — designed to unify quality processes, production workflows, and compliance documentation into one audit-ready system
MedQMS Pro is an integrated purpose-built ERP + QMS platform designed specifically for medical device and healthcare equipment manufacturers. It helps organizations implement and maintain ISO 13485–aligned quality systems by unifying Quality, Production, Inventory, Training, Traceability, and Compliance into one digital platform.
The Problem with Manual QMS & Generic ERP Systems
Excel-based QMS leads to gaps, duplication, and audit stress
Generic ERP doesn’t support ISO 13485 workflows
Quality runs separately from production
Traceability is manual and error-prone
Audit preparation becomes last-minute firefighting


Why MedQMS Pro?


Replaces manual registers & Excel tracking
Complete Traceability from Raw Material to Finished Device
Audit-Ready Digital Records
Get real-time visibility for management decisions
Built for regulated manufacturing. Designed for audits. Loved by operations.
Role-based access & user control
Embed quality into production workflows
MedQMS Pro integrates ISO 13485 workflows directly into production and inventory operations.
Our Solutions
Experience real workflows for quality, traceability, production, and compliance.
Quality Management (QMS)
Incoming & In-Process QC
CAPA and Change Management
Non-conformance Management
QR Code based Complaint Handling
Supplier Quality Evaluation
Audit-ready Digital records
Document & Record Control
SOPs & Controlled Document Management
Version control & approval
Obsolete document management
Controlled access & revision history
Digital work orders linked to QC
Batch & serial number tracking
Material traceability
Production & dispatch monitoring
Production & Process Control
Items Master & Multi-level BOMs
Forward & backward traceability
Batch & Serial number Tracking
Goods receipt with QC
Inventory, Procurement & Traceability
Equipment, Calibration & Maintenance
Equipment Master Records
Calibration Scheduling & Records
Preventive Maintenance
Breakdown Records & History
Audit-Ready Equipment Logs
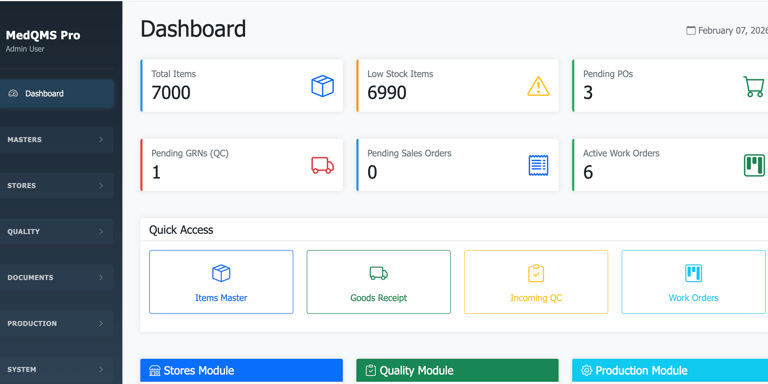
Dashboards & Management Review
Management dashboards
Quality KPIs & trends
CAPA & NC status
Audit findings tracking
QMS performance reports
Who Should Use MedQMS Pro?
Medical Device Manufacturers
ISO 13485/9001 Certified Organizations
Whether you're a Startup, SME, or an established manufacturer, we tailor our support to meet your specific product, process, and team needs.
Startups preparing for regulatory audits
Organizations moving from Excel/manual QMS
Ready to Digitize Your Quality & Compliance System?
Built for audits. Designed for operations.


